-
 IT'S TIME FOR
IT'S TIME FOR
A DIFFERENT APPROACHBrain Trust Bio reimagines ways to make
existing therapies more effective to deliver
patients with central nervous system disease
or injury the highest and best scientific and
medical innovations.
It’s time for a different approach
Imagine you need to send a message to a friend. You could mail them a letter and they might receive it in a few days. You could drive to their home and tell them the news, having to deal with traffic and unforeseen obstacles along the way. But, wouldn’t it be simpler to just pick up your phone and call or text? It’s better. It’s faster.
At Brain Trust Bio, we are giving life-altering medicines a direct route to the body without the roadblocks and side-effects commonly associated with other drug delivery methods.
Our goal is to deliver clinical care breakthroughs for central nervous system (CNS) conditions by reimagining how existing FDA approved therapies are administered and making them safer and more effective. We are leveraging small changes with precision drug delivery to engender massive gains.
An industry leader with a proven track record, Brain Trust Bio has now secured approval to enter into Phase I trials for our Continuous Intrathecal Drug Delivery Method. Building upon successfully treated two patients off-label with no side effects, providing stable and prolonged neuroprotection.We are committed to patients because for people with debilitating CNS disorders—like ALS and multiple sclerosis — every second, minute, hour, week, month or year is more time with loved ones and a step closer to more effective treatments and cures.
For individuals with CNS disorders, the effects can be ravaging. And — with ALS — the typical survival rate is two to five years from the time of diagnosis. During those few short years, those affected by the disease lose abilities we often take for granted like walking, talking, getting dressed, eating independently and driving. They miss moments like birthdays, anniversaries, their child’s graduation or marriage, the birth of grandchildren and other important life events.
At Brain Trust Bio, we are making improvements to drug delivery and enhancing already available treatments to accelerate positive patient outcomes.


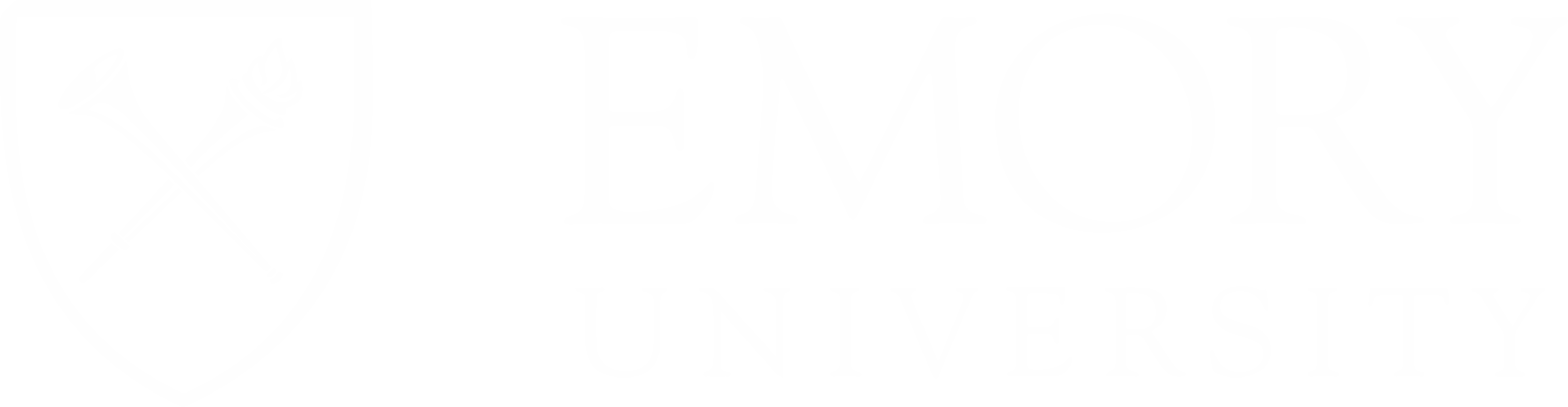


 Our Partners
Our Partners
TRAILBLAZING PARTNERSHIPSS
At Brain Trust Bio, we understand the urgent need to find safe and effective treatments for central nervous system conditions and we understand that we cannot accomplish this on our own. We engage in opportunities to collaborate with cutting-edge firms that are uniquely positioned to leverage our results and support our work. We work closely with these enterprises because we recognize that only by working together will we get to the finish line faster.
 Our Approach
Our Approach
PROOF OF CONCEPT – IMPROVING ON A PROVEN ALS THERAPY
At Brain Trust Bio, we are singularly focused on reformulating and improving the best drugs on the market, based on our successful implementation of riluzole administered by intrathecal infusion (ITRil) for the treatment of amyotrophic central nervous system diseases like lateral sclerosis (ALS). Latest News & Events
Latest News & Events
Brain Trust Bio Receives Approval for Patient Trials Targeting ALS and other CNS Diseases with Continuous Intrathecal Drug Delivery Method
The approval sets the stage for patient trials in Australia designed to enhance treatment efficacy and patient outcomes in the battle against debilitating neurological conditions, and would enable a direct progression to Phase II trials in the U.S. without repetition. ATLANTA, March 25, 2022 – Brain Trust Bio (BTB), a leader in the development of […]
Read More →
Brain Trust Bio Enters Investigational New Drug Enabled Studies for Continuous Intrathecal Drug Delivery Method
ATLANTA, August 1, 2022 — Brain Trust Bio , a next-generation biotechnology company, dedicated to improving the performance of the best medicines for central nervous system (CNS) conditions, today announced it has entered into Investigational New Drug (IND)-enabled studies for its continuous intrathecal (IT) drug delivery method. “The acceptance of our application to enter into […]
Read More →